Dosing and administration
With PrVELPHORO®, you can start your patients on 1 tablet 3 times daily with meals.
Dosing
STARTING DOSE
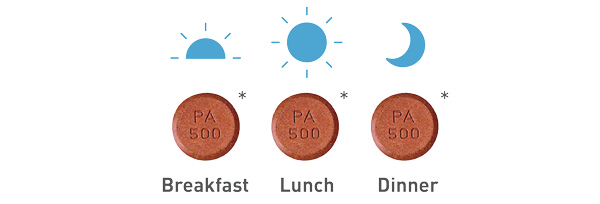
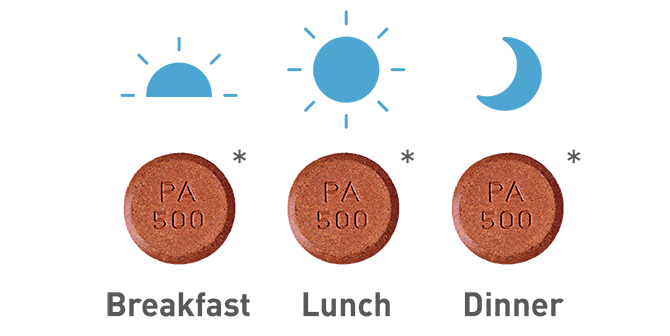
The recommended starting dose of VELPHORO is 1 tablet (500 mg iron) 3 times daily with meals for a total of 3 tablets (1,500 mg iron) per day.
- * Tablet is not actual size
TITRATION AND MAINTENANCE


Titrate VELPHORO dose up or down in increments of 500 mg iron (1 tablet) per day every 2-4 weeks until an acceptable serum phosphorus level is reached, with regular monitoring thereafter.
- * Tablet is not actual size
In clinical practice, treatment will be based on the need to control serum phosphorus levels, though patients who respond to VELPHORO therapy usually achieve optimal serum phosphorus levels at doses of 1,500-2,000 mg iron (3 to 4 tablets) per day. The maximum recommended dose is 3,000 mg iron (6 tablets) per day.1
Serum phosphorus levels must be monitored during titration as needed until an acceptable serum phosphorus level is reached, with regular monitoring thereafter.
Administration
VELPHORO must be chewed and can be broken or crushed to aid with chewing and swallowing.

CHEW
VELPHORO is a chewable tablet. It must be chewed and not swallowed whole.
CHEW
VELPHORO is a chewable tablet.
It must be chewed and not swallowed whole.

BREAK or CRUSH
Tablet may be crushed or broken into small pieces to aid with chewing and swallowing.
BREAK or CRUSH
Tablet may be crushed or broken into small
pieces to aid with chewing and swallowing.
VELPHORO is a woodberry-flavoured chewable tablet and must be taken with meals.1
In order to maximize the adsorption of dietary phosphate, the total daily dose should be divided across the meals of the day, taking into consideration the size of the meals.1
In order to maximize the adsorption of dietary phosphate, the total daily dose should be divided across the meals of the day, taking into consideration the size of the meals.1
- Does VELPHORO interact with other drugs?
- When administering any oral medicinal product that is known to interact with iron, the medicinal product should be administered at least one hour before or two hours after VELPHORO.
- Interaction studies have not been performed in patients on dialysis. Drug-drug interaction studies have been conducted in healthy male and female subjects with losartan, furosemide, digoxin, warfarin, and omeprazole. Concomitant administration of VELPHORO did not affect the bioavailability of these medicinal products as measured by area under the curve.
- Data from clinical studies have shown that VELPHORO does not affect the lipid lowering effects of HMG-CoA reductase inhibitors (e.g., atorvastatin and simvastatin).
- In vitro studies revealed relevant binding of VELPHORO with the following drugs:*
- Alendronate. Take at least 1 hour before taking VELPHORO.
- Doxycycline. Take at least 1 hour before taking VELPHORO or at least 2 hours after taking VELPHORO.
- Levothyroxine. Take at least 1 hour before taking VELPHORO.
- Although no relevant interaction was found in vitro, caution should be exercised when patients take VELPHORO concomitantly with acetylsalicylic acid, cephalexin, cinacalcet, ciprofloxacin, clopidogrel, enalapril, hydrochlorothiazide, metformin, metoprolol, nifedipine, pioglitazone, and quinidine.
- * In vitro interactions are theoretical.
- For more complete dosage and administration information, please refer to the Product Monograph.
- Reference:
- 1. VELPHORO Product Monograph. Otsuka Canada Pharmaceutical Inc.
Important Safety Information
- Clinical use:
- Efficacy and safety in pediatric population (<18 years of age) have not been evaluated.
- No overall differences in safety or efficacy were observed between subjects ≥65 and younger subjects.
- Contraindications:
- Patients who are hypersensitive to this drug or to any ingredient in the formulation, including any non-medicinal ingredient, or component of the container.
- Patients with hemochromatosis or any other iron accumulation disorders.
- Relevant warnings and precautions:
- Diabetes, hereditary fructose intolerance, glucose-galactose malabsorption, and sucrase-isomaltase insufficiency
- Caution in patients with gastrointestinal issues
- VELPHORO may cause black stools which may mask gastrointestinal bleeding
- Patients with hepatic/biliary/pancreatic disorders/disease
- Monitoring and laboratory tests regarding serum phosphorus and iron
- Pregnant or nursing women
- Less common clinical trial or post-market adverse reactions:
- In the 6-week and 55-week clinical studies, tooth discolouration and product taste were some of the less common (<1%), treatment-related treatment emergent adverse events reported in more than one patient.
- Eosinophilic peritonitis has been listed in post-market spontaneous reports.
- For more information:
- Please consult the Product Monograph at https://velphoromonograph.ca for important information relating to adverse reactions, drug interactions, and dosing information which have not been discussed in this piece. The Product Monograph is also available by calling us at 1-877-341-9245.

